The benefits of Health Technology Assessment (HTA) are widely appreciated; well conducted HTAs facilitate patient access to cost-effective technologies that can improve health outcomes, minimise the introduction of ineffective technologies, and contribute to value for money decisions in the context of limited healthcare resources. However, the HTA process does not guarantee the optimum use of scarce healthcare resources post-reimbursement. A drug may prove cost-effective, however if it is used in settings not considered by the HTA then it may no longer be cost-effective. For example, prescribing medicines for unlicensed indications or at higher than recommended doses. Also, continuing to prescribe patent-expired non-exclusive biologic medicines when much lower cost biosimilars are available, does not represent value for money and has significant budgetary implications and opportunity costs.
It is against this background that the Health Service Executive (HSE) is turning to the Medicines Management Programme (MMP) and Health Technology Management (HTM) to ensure ongoing value-for-money prescribing and cost containment. HTM in this context, refers to measures put in place to enhance the safe, effective and cost-effective use of medicines thereby controlling utilisation and expenditure [1]. The MMP was established in 2013, and works in collaboration with the National Centre for Pharmacoeconomics (NCPE), the National Medicines Information Centre (NMIC) and the HSE to provide sustained national leadership relating to medicines management processes, access to medicines and overall expenditure on medicines. The MMP is a team of pharmacists, a data analyst, and a clinical lead, with a wide range of backgrounds and training.
The MMP has undertaken several initiatives aimed at enhancing evidence-based and cost-effective prescribing nationally including the “preferred drugs” initiative, “prescribing and cost guidance” evaluations and “prescribing tips and tools” for appropriate use of medicines. More recently, the MMP has been involved in HTM initiatives resulting in significant impacts on drug utilisation, cost-savings and overall expenditure.
The introduction of evolocumab (Repatha®), a lipid-lowering PCSK9 inhibitor, saw the utilisation of HTA in combination with HTM. Repatha® is licensed in combination with a statin and other lipid lowering therapies for patients unable to reach low density lipoprotein cholesterol (LDL-C) goals with the maximum tolerated statin dose. The HTA for Repatha® was carried out by the NCPE in 2018 and indicated that the drug, which costed over €8,000 per patient per annum, could not be considered cost effective [2]. The budget impact analysis indicated that expenditure could exceed €88 million over five years. As a result of this, after a confidential pricing discount, a managed access protocol (MAP) was introduced in July 2019. Under this MAP, prescribing of Repatha® is confined to designated clinicians who have been approved by the HSE and specific clinical criteria must be met prior to approval. All applications are reviewed by a member of the MMP team. By June 2020, reimbursement had been approved for 77 applications with associated expenditure of less than €400,000 per annum, well below the predicted figures of 900 patients and over €9 million in expenditure. Since then, the number of patients has increased slowly with an average number of 80 patients per month in 2021.
The MMP has also utilised HTM to achieve significant savings in the area of patent-expired non-exclusive biologic medicines adalimumab (originator Humira®) and etanercept (originator Enbrel®). These tumour necrosis factor-alpha (TNF-α) inhibitors were the highest expenditure category on the High-Tech Drug Scheme in 2017, accounting for over €220 million. TNF-α inhibitors are licensed for a variety of inflammatory conditions including rheumatoid arthritis, psoriatic arthritis, inflammatory bowel disease, and plaque psoriasis. The MMP identified preferred biosimilars, known as the best value biologics (BVB) for adalimumab (Imraldi®, Amgevita®, Idacio® and Hulio®) and etanercept (Benapali®) [3].
On 1st June 2019, the HSE introduced a gain-share incentive for prescribers whereby €500 would be made available to the public hospitals when a patient is initiated on or switched to a BVB medicine. In early 2020, the HSE introduced a policy of reimbursing only preferred biosimilars for newly initiated patients on adalimumab and etanercept. Within 12 months the number of patients on biosimilars for adalimumab and etanercept had increased from approximately 100 (as of June 2019) to over 8,000 (as of June 2020) which represents approximately 45% of market share (Figure 1) [4]. Since then, the uptake of BVB medicines has increased steadily to over 60% of market share.
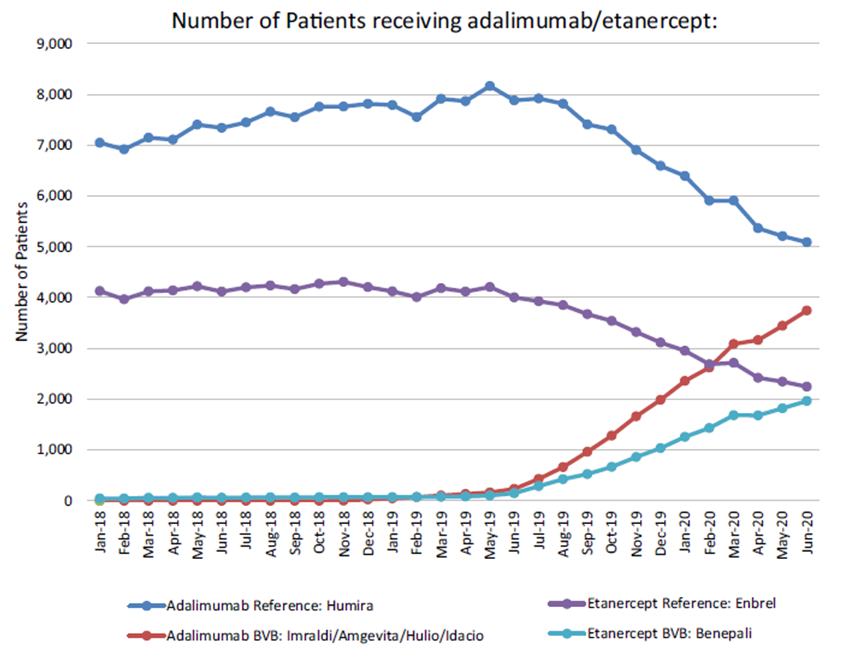
Figure 1. The number of patients receiving adalimumab or etanercept
(originator and BVB medicine).
The barriers to implementation of further HTM measures are largely the time and resources needed to develop, implement, and review such measures. For example, in the case of the BVB medicines, the MMP was aware that a collaborative approach involving clinicians, pharmacists, nurses, patients and the health service would be required to increase utilisation of BVB medicines. Therefore, prior to the selection of the BVB medicines, MMP conducted a 6-week consultation process whereby submissions were invited from relevant stakeholders. The MMP continues to monitor the utilisation and expenditure of BVB medicines.
The MMP is aware that the requirement to complete an online or paper-based application form for medicines under managed access can increase the clinician workload and clinician support is available in that regard. In addition, there may be concern and frustration for patients when the availability of a product is restricted. For example, the introduction of a reimbursement application system for the lidocaine 5% medicated plaster (Versatis®) in September 2017. The MMP published further information for both patients and prescribers to ensure that the rationale for the introduction of this measure was clear and alternative treatment options were highlighted. The future will likely see an increased uptake of HTM in combination with HTA as payers become more aware of the potential to combine value for money assessment with cost containment.
References:
- Smith A, Barry M. Combining health technology assessment and health technology management to deliver cost-effective prescribing and cost containment – the Irish experience. Expert Review of Pharmacoeconomics & Outcomes Research 2020;20:431–6. https://doi.org/10.1080/14737167.2020.1822739
- NCPE. Evolocumab (Repatha®) | National Centre for Pharmacoeconomics n.d. http://www.ncpe.ie/drugs/evolocumab-repatha/ (accessed August 12, 2020).
- MMP. Best-Value Biological Medicines: Tumour necrosis factor-α inhibitors on the High Tech Drug scheme. n.d. https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/best-value-biological-medicines/mmp%20report%20bvb%20medicines%20tnf%20alpha%20inhibitors%20may%202019.pdf (accessed August 6, 2020).
- Duggan B, Smith A, Barry M. Uptake of biosimilars for TNF-α inhibitors adalimumab and etanercept following the best-value biological medicine initiative in Ireland. Int J Clin Pharm 2021. https://doi.org/10.1007/s11096-021-01243-0
Other MMP publications:
- Kennedy C, Smith A, Doran S, Barry M. Sacubitril/valsartan (Entresto) utilisation and prescribing patterns in the context of a reimbursement application system. Br J Clin Pharmacol. 2021 Feb;87(2):406-413. doi: 10.1111/bcp.14393. Epub 2020 Jun 12. PMID: 32470158.
- Smith A, Doran S, Daly M, Kennedy C, Barry M. Effect of an Online Reimbursement Application System on Prescribing of Lidocaine 5% Medicated Plaster in the Republic of Ireland. Appl Health Econ Health Policy. 2021 Jan;19(1):133-140. doi: 10.1007/s40258-020-00586-5. PMID: 32430656.
- Smith A, Barry M. Utilisation, expenditure and cost-effectiveness of cystic fibrosis drugs in Ireland: a retrospective analysis of a national pharmacy claims database. BMJ Open. 2020 Nov 14;10(11):e040806. doi: 10.1136/bmjopen-2020-040806. PMID: 33191267; PMCID: PMC7668370.
- Norris BA, Smith A, Doran S, Barry M. Trends in strong opioid prescribing in Ireland: A repeated cross-sectional analysis of a national pharmacy claims database between 2010 and 2019. Pharmacoepidemiol Drug Saf. 2021 Apr 11. doi: 10.1002/pds.5247. Epub ahead of print. PMID: 33840133.
Useful resources:
- Preferred Drugs: https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/preferred-drugs/
- Prescribing and Cost Guidance: https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/guidance/
- Prescribing Tips and Tools: https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/prescribing-tips-and-tools/
- Managed Access Protocols: https://www.hse.ie/eng/about/who/cspd/ncps/medicines-management/managed-access-protocols/
 | Dr Amelia Smith is a data analyst at the Health Service Executive’s Medicines Management Programme. She is a graduate of Biomedical Sciences, Maynooth University and holds a Masters degree in Translational Oncology from Trinity College Dublin. In 2014, Amelia joined the SPHeRE programme as a PhD scholar under the supervision of Prof Kathleen Bennett. Funded by the Irish Cancer Society’s Breast-Predict collaboration, she was awarded a PhD from Trinity College Dublin in 2019. Amelia’s thesis was titled ‘Pharmacoepidemiological studies of breast and colorectal cancer: The association between statins and cancer outcomes’ and utilised pharmacy claims data linked to cancer registry data to investigate the association between statin medicines and cancer outcomes. Amelia is a past-chair of the International Society for Pharmacoepidemiology student council. Research interests include drug utilisation, population health and health services research, and pharmacoeconomics. |

